Researchers from the Instituto de Investigaciones Biomédicas Alberto Sols (IIBM) CSIC-UAM and CIBERdem, led by Dr. Ángela Martínez-Valverde, have shown that olanzapine, an antipsychotic drug widely used in clinical practice, regulates body weight and energy balance in male mice.
The results of this study, published in the journal Metabolism, could be relevant in clinical practice to attenuate the adverse side-effects associated with chronic treatment with this second generation antipsychotic drug.
Second-generation antipsychotic drugs are the mainstay choice for the treatment of schizophrenia and other psychiatric disorders. However, numerous studies have shown a variety of metabolic side-effects such as obesity, insulin resistance as well as dysfunctions on glucose and lipid metabolism. These alterations from the beginning of the treatment may lead to severe complications, as type 2 diabetes and cardiovascular disease.
Dr. Martínez-Valverde, a Principal investigator and group leader at the Instituto de Investigaciones Biomédicas Alberto Sols (IIBM) CSIC-UAM and also at the Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERdem), has led a study showing substantial differences in the regulation of body weight and energy balance in male mice treated with olanzapine, an antipsychotic drug widely used in clinical practice. The results published in the journal Metabolism, show how the effects of the drug might depend on the administration route: mice that received olanzapine supplemented in the diet presented hyperphagia, weight gain and decreased energy expenditure; while, surprisingly, those receiving olanzapine via intraperitoneal injection lost weight concomitant with increased energy expenditure.
"We studied the hypothalamus of mice treated with olanzapine via intraperitoneal injection and found that the phosphorylation of AMP-dependent protein kinase (AMPK) was decreased and that this effect was responsible for the activation of brown and white subcutaneous inguinal - also called beige - adipose tissue depots of these mice, were we found increased levels of the uncoupling protein UCP-1" comments the first author of this article, Vitor Da Silva Ferreira. "It is also important to highlight that the same molecular changes were observed when we administered olanzapine via intrahypothalamic injection. And the direct link between these two effects was evidenced in mice that overexpress a constitutively active form of AMPK specifically in the hypothalamus, which prevented the activation of the fat depots" continues the young researcher.
In contrast, oral administration of the drug olanzapine to mice did not promote modulation of the hypothalamus-brown/beige adipose tissue axis, and those mice gained weight in a similar progression to what is described for the human patients.
Analysis of olanzapine levels after a single administration of olanzapine via intraperitoneal injection or via oral gavage have shown that the levels of the drug in the plasma and hypothalamus were 2.5-fold higher than in mice intraperitoneally treated. This result, and others described in this article, suggest that olanzapine levels reaching the hypothalamus could be determinant for the modulation of AMPK phosphorylation in this brain region and, as consequence, for the activation of thermogenesis in brown and beige adipose tissues preventing weight gain.
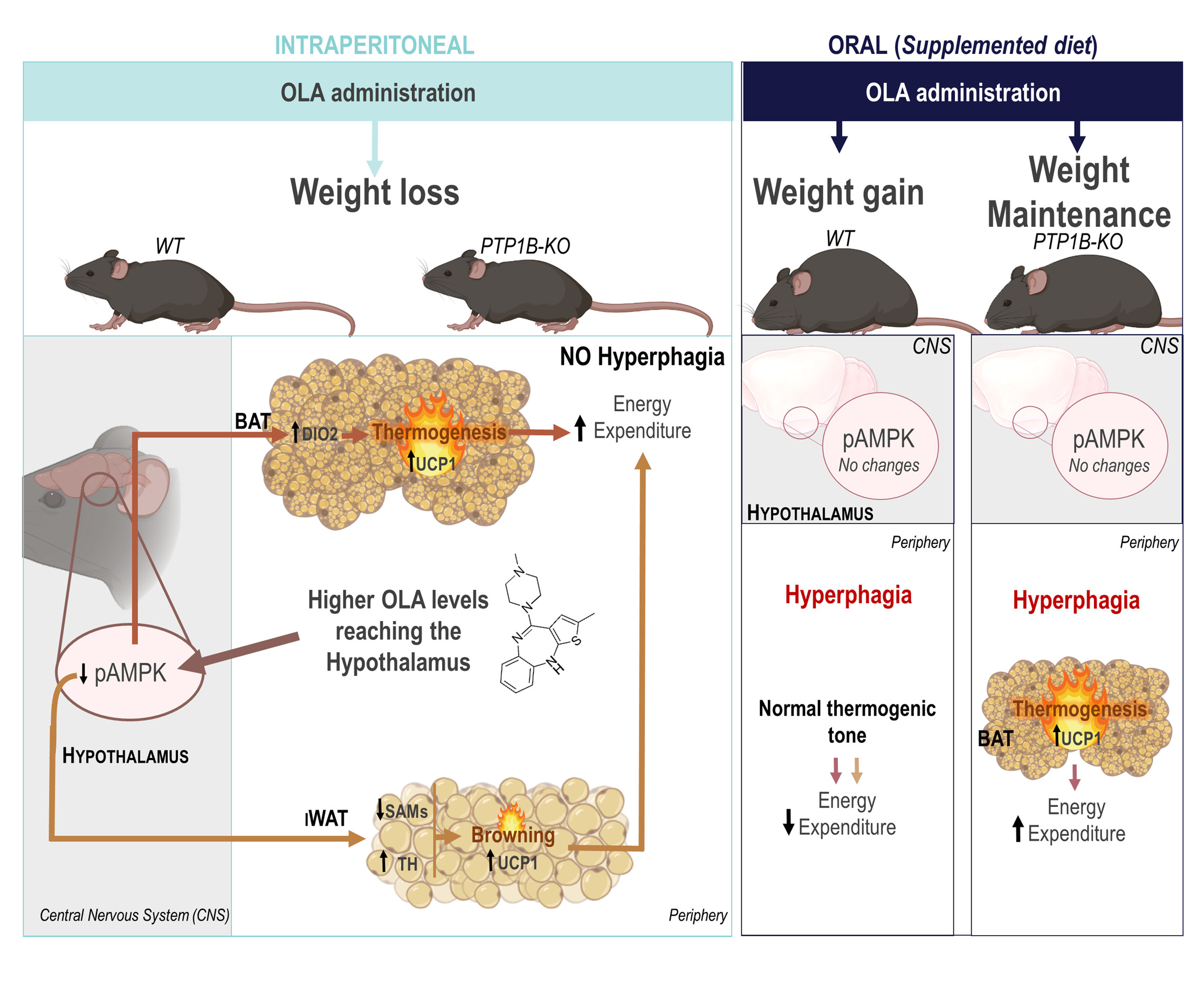
"It is important to highlight that our study has shown that the inhibition of protein tyrosine phosphatase 1B (PTP1B), a therapeutic target for type 2 diabetes and obesity, also confers protection against weight gain induced by olanzapine oral administration, by promoting the activation of brown adipose tissue and increasing energy expenditure" concludes Dr. Martínez-Valverde.
In addition to the IIBM groups that participated in this innovative work, also researchers from the Instituto de Investigación Sanitaria La Princesa, the Centro Nacional de Investigaciones Cardiovasculares (CNIC) and the University of Uppsala in Sweden have collaborated and contributed very importantly to this study.