- The group led by Dr. Manuel Izquierdo has participated in a pioneering study on the immune synapse produced by tandem CD19/CD22 CAR-T cells, successfully applied as a new CAR-T therapy in pediatric patients with leukemia.
The Nanoimmunology and T Lymphocytes group, led by Dr. Manuel Izquierdo at the Instituto de Investigaciones Biomédicas Sols-Morreale (IIBM), CSIC-UAM, has contributed to the basic characterization studies of the new tandem CD19/CD22 CAR-T, designed and clinically applied by Dr. Antonio Pérez-Martínez from the Unidad de Investigación y Terapias Avanzadas del Hospital Universitario La Paz. This therapy, developed in collaboration with La Fundación Cris Contra el Cáncer, has been successfully used in a group of ten patients, as recently published in eBioMedicine (The Lancet.)
The research carried out by Dr. Izquierdo focused on analyzing the structure and function of the immune synapses generated by CD19/CD22 CAR-T cells, as preliminary studies prior to their therapeutic application (see Figure 1).
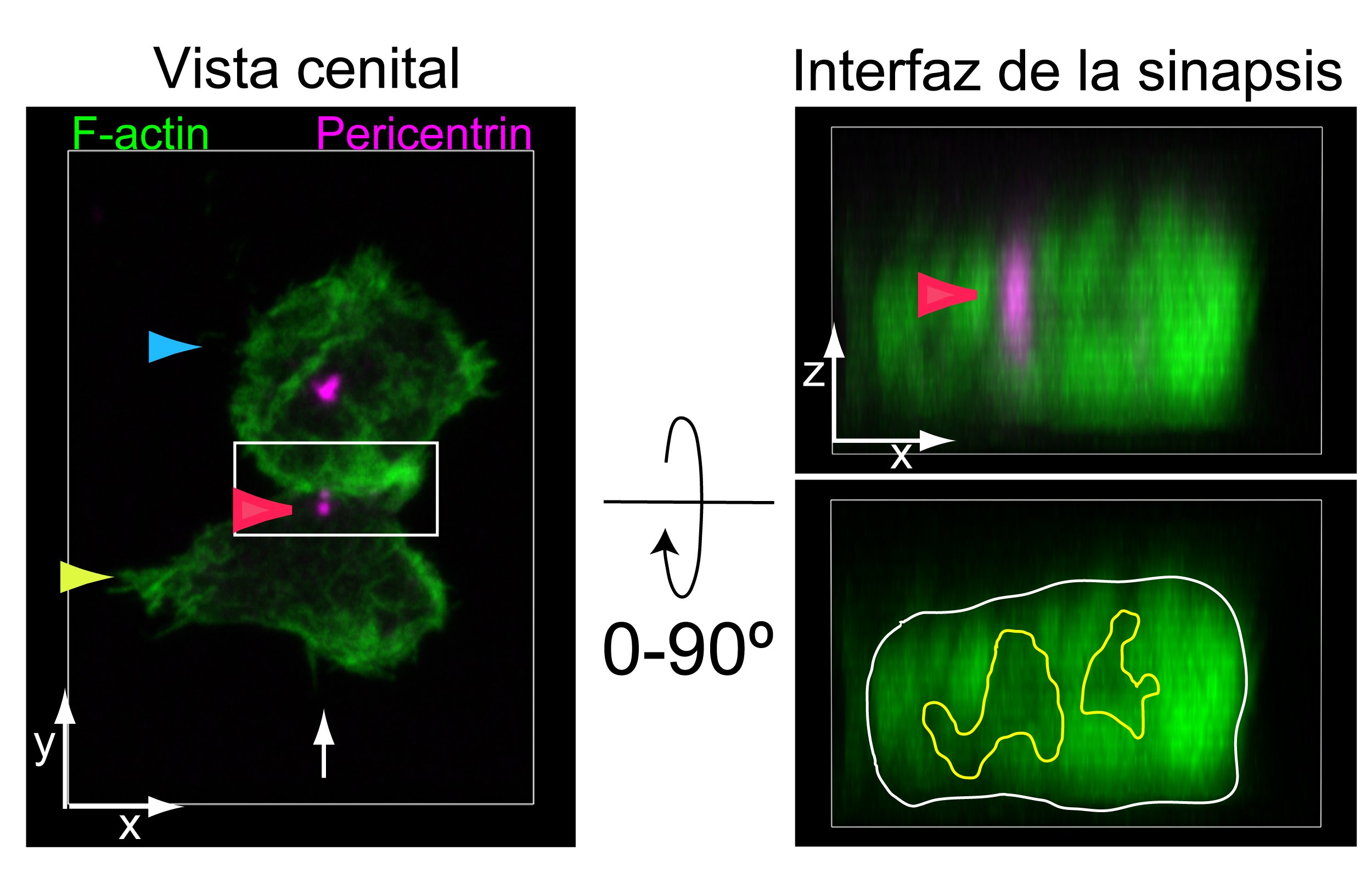
Figure 1: Tandem CD19/CD22 CAR-T cells (yellow arrow) were co-cultured with CD19+/CD22+ Raji tumor cells (blue arrow). Cell conjugates were fixed and labeled to visualize F-actin (green) and the centrosome using an anti-pericentrin antibody (magenta). Synapses were visualized by confocal microscopy: on the left, the top view of the immune synapse is shown; on the right, after a 90° rotation, the immune synapse interface (white rectangle) is displayed. The multifocal architecture of the synapse developed by tandem CAR-T cells and the position of the centrosome (red arrow) can be observed.Courtesy of Javier Ruiz-Navarro, Alfonso Navarro-Zapata, Antonio Pérez-Martínez, and Manuel Izquierdo.
The study involved pediatric and young adult patients with high-risk B-cell acute lymphoblastic leukemia (B-ALL), all of whom lacked conventional therapeutic alternatives. The administration of this therapy was performed as a last option, under a “compassionate use” program, supported in part by previous basic studies conducted by Dr. Izquierdo’s research group.
CAR-T therapy
Adoptive immunotherapy with CAR-T cells (T lymphocytes modified in the laboratory to detect and destroy tumor cells) consists of equipping the patient’s T lymphocytes with a chimeric antigen receptor (CAR) through molecular biology techniques (Figure 2). This receptor combines two essential functions: the specific recognition of a tumor antigen and the activation of an effective immune response.
After the formation of the immune synapse—the cellular basis of the immune response corresponding to the “recognition” phase in Figure 2—the CAR receptor enables T lymphocytes to accurately identify a specific tumor target and trigger a targeted cytotoxic response. This response is evaluated by the repositioning of the centrosome toward the synapse.
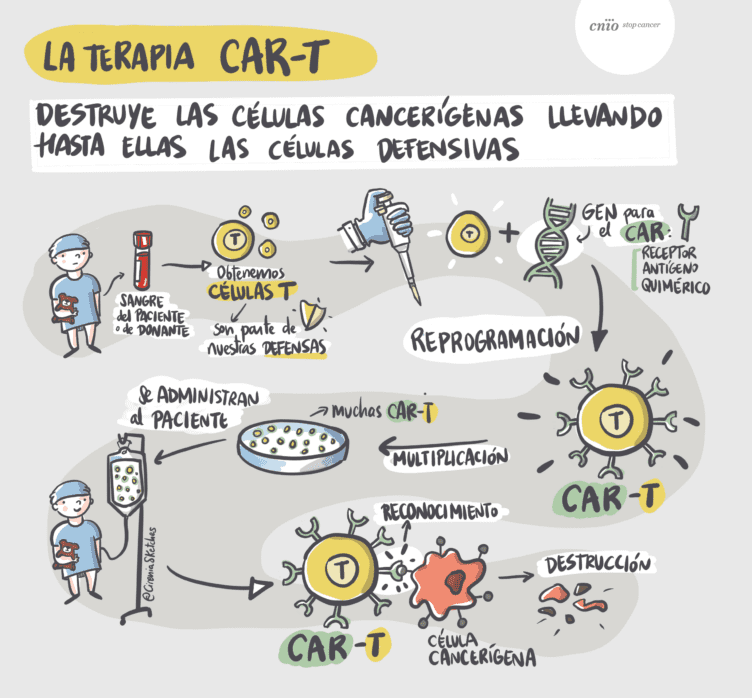
Figure 2. Courtesy of Antonio Pérez-Martínez, Fundación CNIO-Cirenia sketches
Currently, CAR-T therapy is particularly effective against hematological malignancies (lymphomas and leukemias). However, some tumors manage to evade immune control by losing the tumor antigen recognized by the CAR, thereby limiting its efficacy. As a consequence, more than half of the patients treated with CAR-T therapy experience relapses after an initial favorable response or even after being considered disease-free.
To overcome this limitation, the new strategy developed at Hospital Universitario La Paz uses a tandem CAR-T capable of simultaneously recognizing two different molecules present on tumor cells (CD19 and CD22). Thus, if the tumor stops expressing one of them, CAR-T cells can still detect the other, reducing the likelihood of relapse.
This approach represents the first clinical application in Spain of this therapeutic strategy — with precedents published in China, the United States, and the United Kingdom — and is the result of a close translational collaboration between basic scientists and clinical researchers.
Article references
Ruiz-Navarro J, Fernández-Hermira S, Sanz-Fernández I, Barbeito P, Navarro-Zapata A, Pérez-Martínez A, Garcia-Gonzalo FR, Calvo V, Izquierdo Pastor M. Formin-like 1β phosphorylation at S1086 is necessary for secretory polarized traffic of exosomes at the immune synapse in Jurkat T lymphocytes. eLife. 2024;13:RP96942. doi:10.7554/eLife.96942
González-Martínez B, Galán-Gómez V, Navarro-Zapata A, Mirones-Aguilar I, Cobo M, Pernas-Sánchez A, Vallejo S, Sánchez-Zapardiel E, León-Triana O, Echecopar C, Martínez-Romera I, Guerra-García P, San Román-Pacheco S, Escudero A, Izquierdo E, Izquierdo M, Naharro S, Martín-Ayuso A, Bareke H, París-Muñoz A, et al. Tandem CD19/CD22 CAR T-cells as potential therapy for children and young adults with high-risk r/r B-ALL. EBioMedicine. 2025;118:105872. doi:10.1016/j.ebiom.2025.105872
Cover image: Co-culture of T cells with Raji tumor cells (blue) loaded with superantigen and labeled for F-actin (green). In the center of the image, an immune synapse is established between a Raji cell (top) and a T cell (bottom) stimulated through the antigen receptor. The synapse is characterized by a circular accumulation of F-actin at the contact zone and an F-actin-free area in the center.