- The group led by Dr. Margarita Díaz-Guerra has discovered a new mechanism involved in the destabilization of the Golgi apparatus after ischemic stroke, and its suitability as a therapeutic target for treatment of this form of brain damage
The research group Herramientas Diagnósticas y de Neuroprotección en Excitotoxicidad e Isquemia Cerebral, led by Dr. Margarita Díaz-Guerra at “Instituto de Investigaciones Biomédicas Sols-Morreale” (IIBM, CSIC-UAM), has published a study in the journal Cell Death & Disease that unveils an important regulatory mechanism of neuronal damage in ischemic stroke. Furthermore, this work demonstrates that a neuroprotective peptide previously developed by the group which was successfully tested in preclinical models of this form of brain damage (https://doi.org/10.15252/emmm.201809950) precisely interferes with this newly discovered pathological mechanism.
Stroke is very important from social, healthcare, and economic perspectives, primarily due to its high associated mortality and morbidity rates, being the leading cause of disability in adults and the second leading cause of dementia. Therapies aimed at restoring blood flow are highly relevant for ischemic stroke, although they can only be applied to a minority of patients. “Currently, novel therapeutic strategies are focused on trying to rescue the ischemic penumbra area, a tissue surrounding the irreversibly damaged zone known as the infarct core”, explains Dr. Gema Esteban Ortega, first author of this article. “It is critical to act quickly and prevent neurons in the penumbral area from secondarily dying due to a mechanism known as excitotoxicity, which, among other things, profoundly disrupts neurotrophin-mediated support needed for neuronal survival”.
The group's previous work had shown that processing of neurotrophin receptor TrkB-FL was secondary to its endocytosis and played a central role in the processes of excitotoxicity and cerebral ischemia. Therefore, as a working hypothesis, the group proposed that TrkB-FL maintenance on the cell surface, and subsequent receptor stabilization, might be used as a target for the design of stroke therapies. As explained by Margarita Díaz-Guerra, in order to test this hypothesis, they designed a type of peptides, denominated cell-penetrating peptides, containing different short TrkB-FL sequences belonging to a protein region involved in recycling. One of these peptides was able to cross the blood-brain barrier, a main challenge for brain treatment, and interfere with TrkB-FL degradation in excitotoxicity and ischemia. This result correlated with decreased receptor endocytosis and reduced neuronal death under excitotoxic conditions, as well as with a significant decrease in infarct volume and neurological improvement in a preclinical model of stroke.
The published work now deepens into the mechanism of TrkB-FL regulation induced by excitotoxicity demonstrating that, after endocytosis, receptor interaction with endosomal protein Hrs is promoted, followed by TrkB-FL retrograde transport to the Golgi apparatus, where it is processed by action of calpain proteases. Interestingly, this process is concomitant with a dramatic disruption of the Golgi apparatus, observed both in cultures of cortical neurons subjected to excitotoxicity and brain tissue adjacent to the infarct core. Dr. Elena Torres Campos, also an author of this work, highlights the importance of this finding, since disruption of this organelle is a common and central event in many neurodegenerative processes, in addition to stroke.
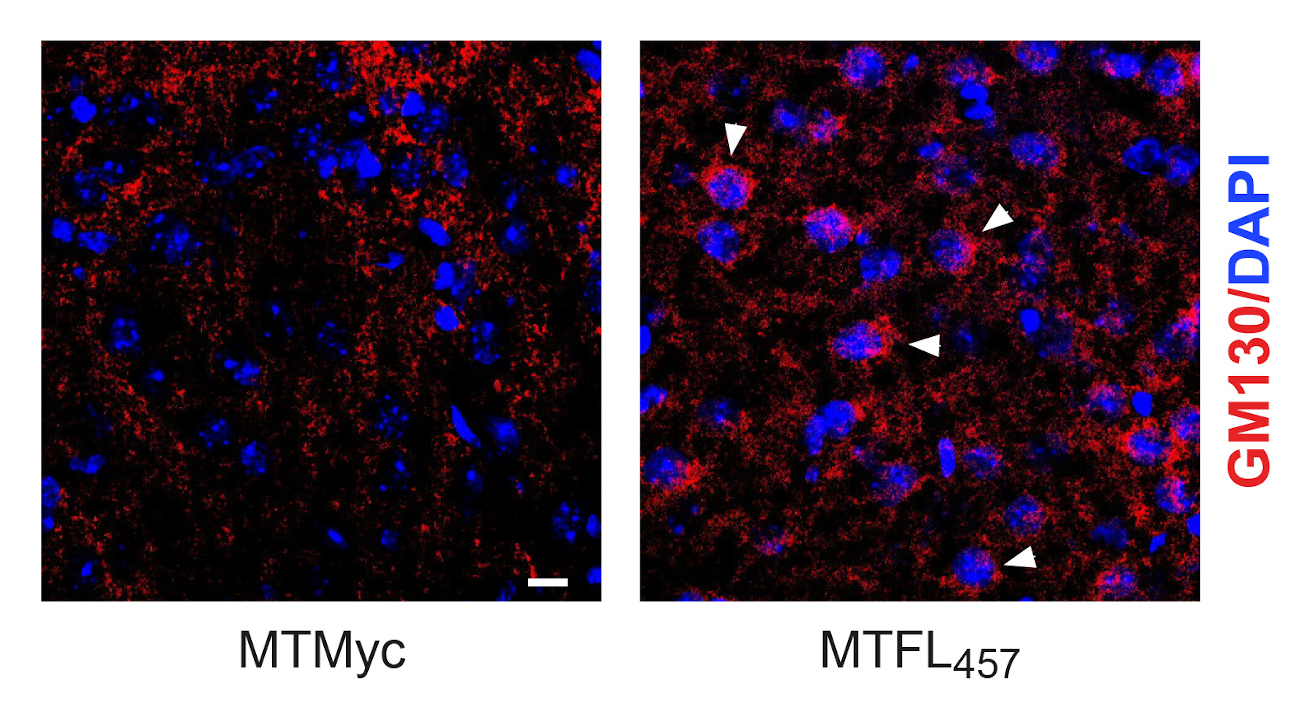
Stabilizing effect of neuroprotective peptide MTFL457, containing a short TrkB-FL sequence, on the Golgi apparatus. In regions adjacent to the infarct core of mice subjected to ischemia, an antibody for Golgi specific protein GM130 (red) shows a better-preserved organelle’s structure in animals treated after injury with MTFL457 (arrowheads) compared to those treated with a control peptide (MTMyc)
Finally, this work also demonstrates that the TrkB-FL-targeted neuroprotective peptide not only interferes with TrkB-FL/Hrs interaction and TrkB-FL retrograde transport and degradation in the Golgi apparatus, but also prevents destabilization of the organelle itself after stroke. Therefore, TrkB-FL not only plays a central role in Golgi fragmentation taking place in neurodegenerative diseases but also constitutes a promising therapeutic target for their treatment.
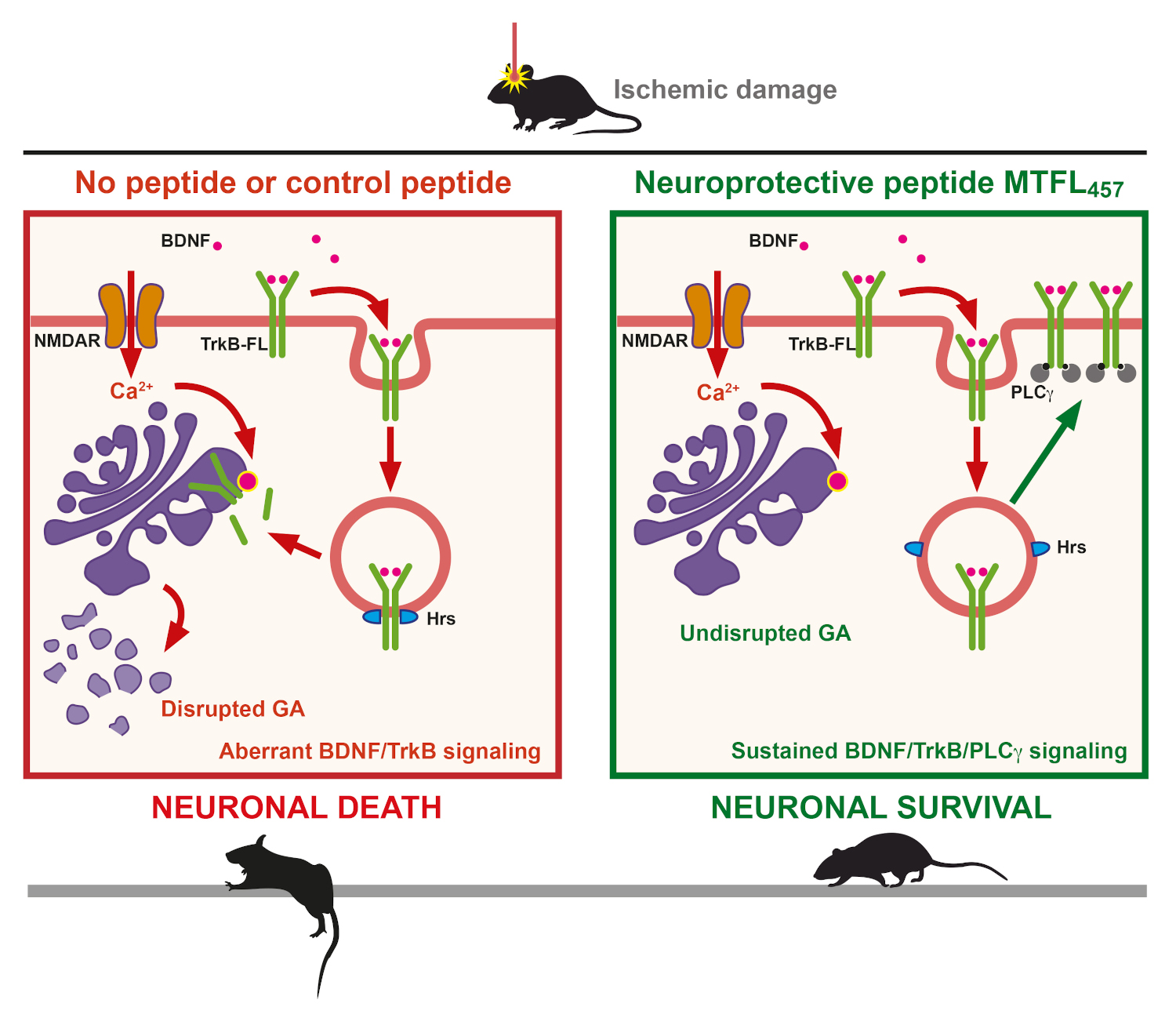
Proposed model for the action of neuroprotective peptide MTFL457 containing specific TrkB-FL sequences
The work has been funded by Agencia Estatal de investigación (PID2019-105784RB-100 y PID2022-137710OB-I00).
Article reference: Gema M. Esteban-Ortega, Elena Torres-Campos and Margarita Díaz-Guerra. Retrograde transport of neurotrophin receptor TrkB-FL induced by excitotoxicity regulates Golgi stability and is a target for stroke neuroprotection. Cell Death & Disease 16, 659 (2025). https://rdcu.be/eC6oY
Cover image: Primary culture of cortical neurons subjected to excitotoxicity showing colocalization of TrkB-FL (red) and the Golgi apparatus marker protein GM130 (green), this organelle presenting signs of fragmentation.